Explain the Difference Between a Mixture and a Solution
The key difference between solutions and mechanical mixtures is that solutions contain dissolved substances whereas mechanical mixtures do not. Use the particle theory to explain.

What S The Difference Between Heterogeneous And Homogeneous Mixtures Examples Of Mixtures Heterogeneous Mixture Homogeneous Examples
Generally we need both mixtures and pure substances for different purposes in our life.

. Sand oil and water and chicken noodle soup are examples of heterogeneous mixtures. This is normal though. A solution is a homogeneous mixture of two or more substances.
Mixture contains two or more substances which are not chemically combined. Which molecules are polar. A solution is a solid the solvent dissolved in a liquid the solute.
Maharashtra State Board SSC English Medium 7th Standard. They have no physical interactions. Matter can be classified as pure substances and mixtures.
The difference between a solution and a mechanical mixture. Mixture comprises two or three compounds that arent fused chemically. Mixtures Are Either Homogeneous or Heterogenous.
A solution is a type of mixture. Solution is also a type of mixture but due to innumerable differences between them solution and mixture are often considered separate. A compound is a substance created by when two or more chemical elements are bound together.
The mechanical mixture has been the physical mixing of the substancesThe components in the mixture are differenced from one anotherThe mixture components have. Correct answer to the question Explain the difference between a mixture and a solution. Heterogeneous mixtures homogenous mixtures Mixture mixtures solutes solution.
What is the difference between a mixture a solution and a pure substance. Difference Between Mixture and Solution Mixture vs Solution In chemistry some terminologies and definitions can be matched up mostly if our brain doesnt have too much information overload. In this mechanical mixture.
In a solution substances are dissolved completely and they cannot be filtered out. Concept Notes Videos 204. N Using the solubility rules will the following molecules be soluble or insoluble in water.
Correct answer to the question I NEED HELP PLEASE THANKS Explain the difference between a homogeneous mixture and a heterogeneous mixture. Milk is a mixture of liquid butterfat. Chemically all matter is either a pure substance an element or compound or a mixture of two or more elements andor compounds.
A mixture of salt and water c. Mixture has been defined as the combination of two or more substancesThe solution has been given as the completely dissolved substances. Difference between molecule and mixture is something we need to know when we are examining the concept of matter.
CBSE Previous Year Question Paper With Solution for Class 12 Arts. The differences between the mixture and solution can be listed as follows. In general mixtures combine substances that neither bond to one.
An example of a colloid is milk. A solution appears the same throughout. Since sugar and salt look alike it may look like one substance.
Describe whether each of the following is a solution or not. Read on for a simple explanation of the difference between solutions suspensions and colloids all of which can be accurately classified as mixtures. Give an example of each.
Explain the difference between a solution and mixture 2. In chemistry though there should be no excuse. Question Bank Solutions 1973.
The components of a solution do not separate when left standing and cannot be separated by filtration. The difference is the appearance at the macroscopic level the level we see it at. A heterogeneous mixture appears to be made of different substances.
Its a mixture because the pieces of the different vegetables can be retrieved by a physical method such as picking them with a fork. A heterogeneous mixture is two substances in different states ie. Difference in Mixture and solution.
They only have physical interactions. Imagine you have a mixture of sugar and salt. The components of mixture are never fused or interacted chemically whereas in solution they may or may not interact chemically.
Explain the difference between a polar and nonpolar molecule. Throw a spoonful of sugar into a cup of water and stir until theres no sugar left on the ground of the cup. In the fluid phase gas or liquid or any combination of those a solution is transparent thought not colourless.
A mixture is a substance composed of two or more matter that can be separate with the help of physical methods. In a mixture substances are generally just mixed and are not completely dissolved. Through combining two or more substances a mixture is produced.
The difference between a pure substance and a mixture. Solutions have a solute and a solvent. Mechanical mixture or heterogeneous mixture a mixture with different parts that you can see TURN Other mechanical mixtures are not as easy to see.
This makes the solution look like one. Particles in a solution are mixed together evenly. Mastery is always the key.
If you look closely you can see the different crystals. When we are trying to recall we tend to mix concepts and such. Mixture or solution.
Salt sand and water are an example of a mechanical mixture. Give reasons for your choice. A gas and a.
Difference Between Mixture and Solution. The components of a solution do not separate when left standing and cannot be separated by filtration. A mixture of clay and water b.
Homogeneous mixtures are sources of water saline solution some alloys and bitumen. They appear very similar to solutions but the particles are suspended in the solution rather than fully dissolved. The difference between a colloid and a suspension is that the particles will not settle to the bottom over a period of time they will stay suspended or float.
The key difference between solutions and mechanical mixtures is that solutions contain dissolved substances whereas mechanical mixtures do not. Explain the Difference Between Mixture and Compounds. What is the difference between a solution and mechanical mixture.
A homogeneous solution tends to be identical no matter how you sample it.

Mixtures Solutions Youtube Matter Science Solutions And Mixtures Science Videos

Illustration Of Heterogeneous And Homogeneous Mixtures Homogeneous Mixture Heterogeneous Mixture Mixtures

Use This Product To Do A Quick And Simple Practice Or Assessment Of Your Student S Understanding Of Th Chemistry Classroom Compounds Science Teaching Chemistry

What Is A Heterogenous Mixture Get A Clear Definition And Examples Heterogeneous Mixture Party Mix Food Coloring Pages

Compound Vs Mixture Difference And Comparison Diffen Compounds Science Teaching Chemistry Chemistry Classroom

Mixtures And Solutions Powerpoint 3rd 5th Grade Science Powerpoint Powerpoint Solutions

Mixtures And Solutions Tictactoe Extension Activities Physical Science Lessons Matter Science Science Curriculum

Pin By Sarah Fariss On Science Solutions And Mixtures Science Anchor Charts 8th Grade Science Science Notes

Learn About Solutions Science For Kids Teaching Science Science For Kids Chemistry Classroom

Supersaturated Solution Easy Science Chemistry Experiments Solutions Easy Science

Let S Mention The Difference Between A Solution And A Heterogeneous Mixture A Solution Is A Comb Chemical Science Solutions And Mixtures Heterogeneous Mixture

Solute And Solvent Dissolving Matter Science Chemistry For Kids Solutions

Science Posters And Anchor Charts Vol 1 Science Chemistry Teaching Chemistry Homeschool Science
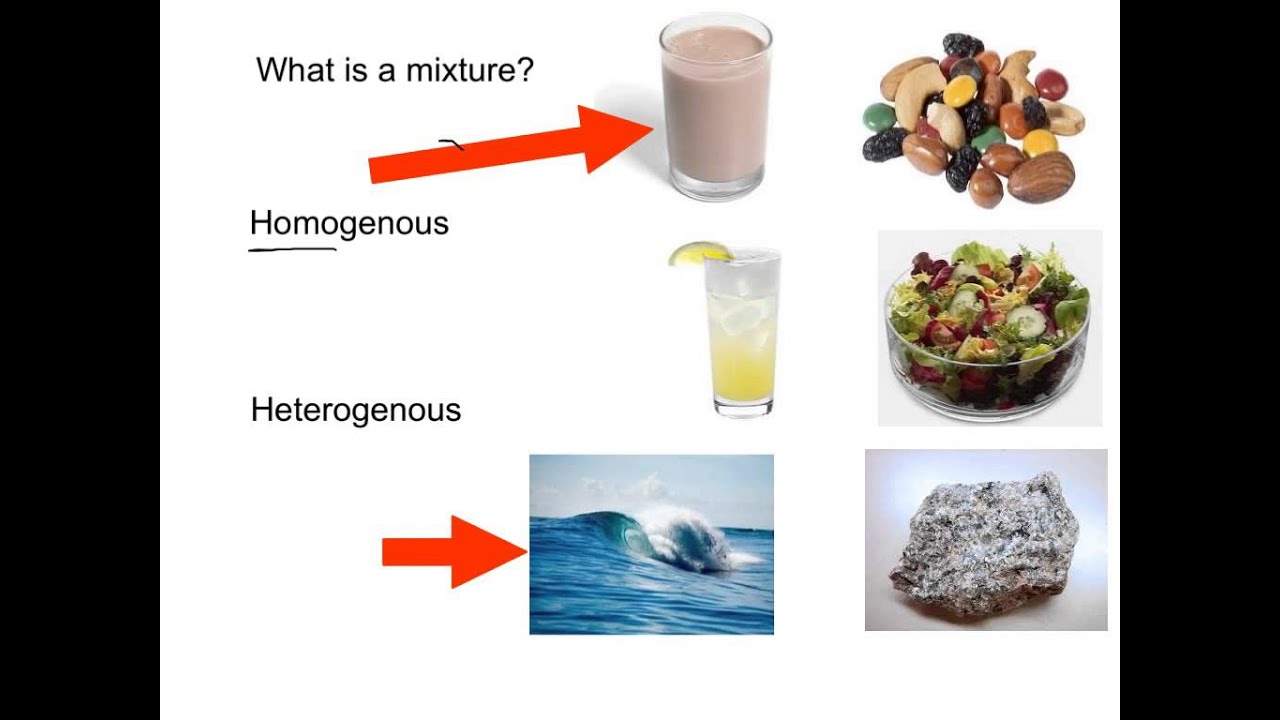
Homogenous And Heterogenous Mixtures Heterogeneous Mixture Matter Science Teaching Middle School Science

Homogeneous Solution Of Water And Salt And Heterogeneous Mixture Of Water And Sand In Glass Beakers Chemist Chemistry For Kids Heterogeneous Mixture Chemistry

Homogeneous And Heterogeneous Mixture Heterogeneous Mixture Homogeneous Mixture Mixtures

Pure Substances And Mixtures Venn Diagram Examples Venn Diagram Worksheet Venn Diagram Template


Comments
Post a Comment